barostim neo system
BAROSTIM NEO System is indicated for the improvement of symptoms of heart failure quality of life six-minute hall walk and functional status for patients who remain symptomatic despite treatment with guideline-directed medical therapy are NYHA Class III or Class II who had a recent history of Class III have a left ventricular ejection fraction 35 a. Tesi di laurea magistrale.

Barostim Neo Neuromodulation Implantable System Usa
Dispositivi impiantabili per lattivazione del riflesso barocettivo Rheos Barostim neo e denervazione renale Symplicity TM system FARMACIA DM.

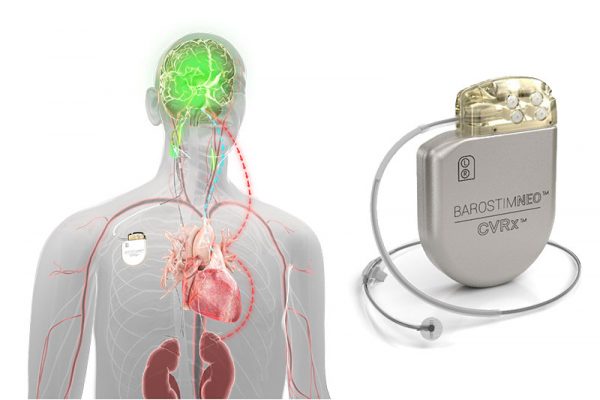
. The Barostim Neo System includes a pulse generator that is implanted below the collar bone and is connected to a lead that attaches to the carotid artery in. The CY 2021 OPPSASC Payment System final rule with comment period would further advance the agencys commitment to strengthening Medicare and reducing provider burden so that hospitals and ambulatory surgical centers can operate with increased flexibility and patients are better equipped to be active healthcare consumers. Short-Mid-Term Fu of New COC36 patients 180.
27004 Dipartimento di Scienze della Vita. Extended Phase BeAT-HF PAS 178. Le nuove frontiere terapeutiche nel trattamento dellipertensione resistente.
BAROSTIM NEO System is indicated for the improvement of symptoms of heart failure quality of life six-minute hall walk and functional status for patients who remain symptomatic despite treatment with guideline-directed medical therapy are NYHA Class III or Class II who had a recent history of Class III have a left ventricular. DEPUY CERAMAX CERAMIC TOTAL HIP SYSTEM.

Fda Approves Device To Treat Patients With Heart Failure Wjar

Barostim Neo Neuromodulation Implantable System Usa

Interventional Treatment For Heart Failure Receives Fda Approval Biba Medtech Insights
About Us Cardiovascular Interventions

Baroreflex Activation Therapy Study In China 2022 Wiki English

Barostim Neo Electrical Stimulator Approved For Heart Failure In Europe Video Medgadget

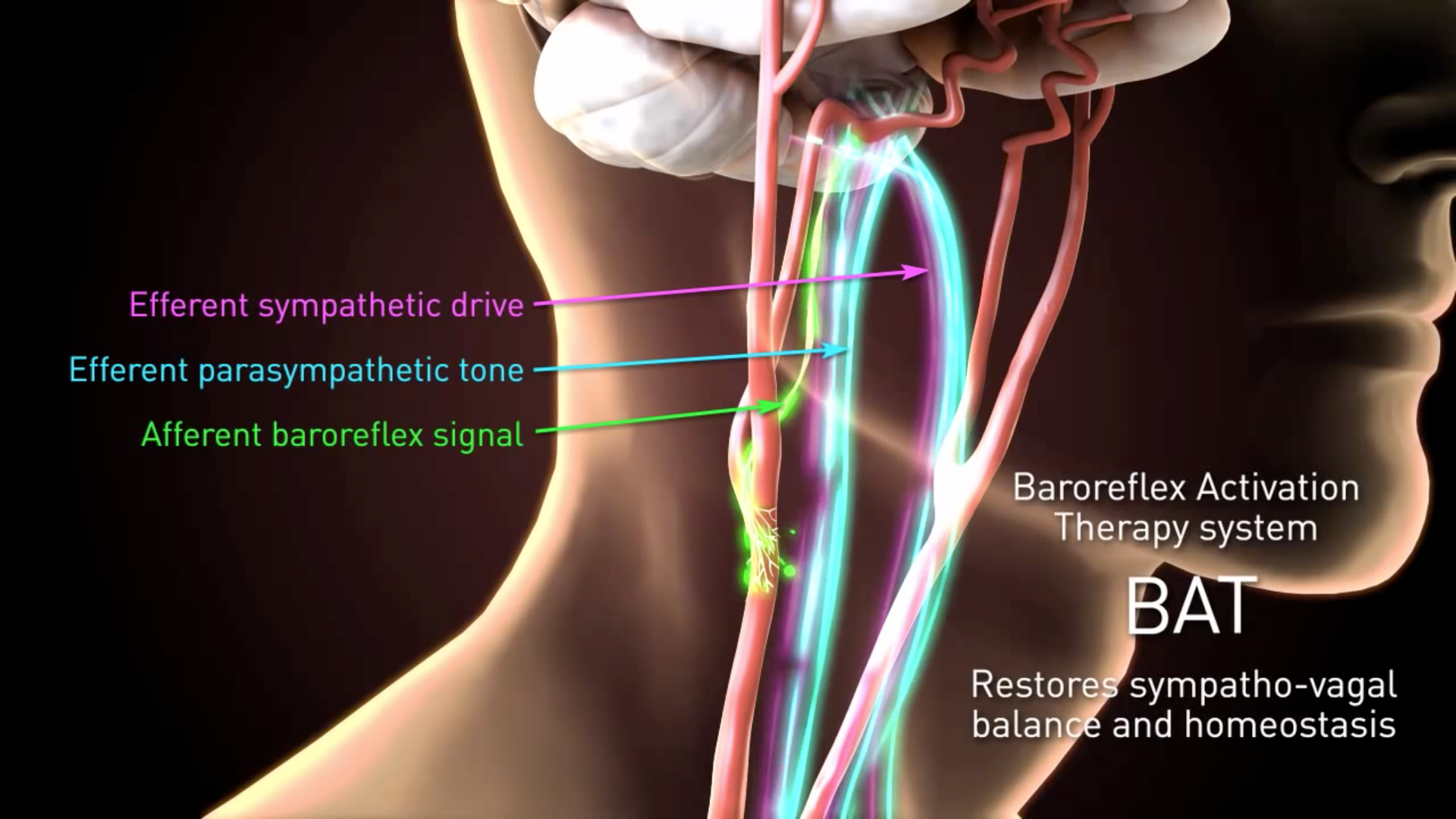
Baroreflex Activatie Therapie Ppt Download

Fda Approves Device To Treat Patients With Heart Failure Wjar

Uofl Health First In Kentucky To Offer Barostim Neo Device For Heart Patients Whas11 Com

Baroreflex Activation Therapy Barostim Neo System Tctmd Com
Barostim Neo 1 Radcliffe Cardiology

First Rheos And Second Barostim Neo Generation Baroreflex Download Scientific Diagram
First Implant Made For Barostim Neo Device To Treat Hypertension Daic

Cvrx Barostim Neo Now Cleared For Mri Use In Europe Medaxs

Baroreflex Activation Therapy By The Rheos System And The Barostim Download Scientific Diagram

Barostim Neo Neuromodulation Implantable System Usa

Fda Approves Device To Treat Patients With Heart Failure Wjar
Cvrx S Barostim Neo Gets Ce Mark For Use With Mris Massdevice

Comments
Post a Comment